Processing...


Disha Diagnostic Centre
+91-2162235580/23338
Nandadeep Pathology
(02162) 239237/7219831971
Nirmay Hospital
(02162) 299158/233181
Jeevanjyot Laboratory
(02162) 233927
PRINCIPLE: In rare instances, a Barr body smear may be obtained from the upper inside cheek, a buccal smear, to determine if Barr bodies are present. A properly collected cheek scraping enables the cytology department to identify the presence of Barr bodies.
PRINCIPLE: To collect a breast specimen which will allow for cytologic preparation and evaluation at the Laboratory.
PRINCIPLE: To collect a bronchial specimen which will allow for cytologic preparation and evaluation at the Laboratory. The most useful cytologic technique is usually direct brushings of a visible lesion.
PRINCIPLE: To collect a bronchial specimen which will allow for cytologic preparation and evaluation at the Laboratory. The most useful cytologic technique is usually direct brushings of a visible lesion.
PRINCIPLE:To collect a bronchoalveolar lavage (BAL) specimen which will allow for cytologic preparation and evaluation at the Laboratory.
PURPOSE: Commonly a bronchoalveolar lavage specimen is collected to identify organisms such as Pneumocystis carinii, and bacterial, viral, or fungal agents causing pulmonary infections.
PRINCIPLE: To collect a cerebrospinal fluid (CSF) which will allow for cytologic preparation and evaluation at the Laboratory. CSF may be a lumbar puncture fluid from central canal of spinal cord, fluid from the ventricles of the brain, or occasionally from a shunt inserted to relieve hydrocephalus.
PRINCIPLE: To collect a gastrointestinal specimen which will allow for cytologic preparation and evaluation at the Laboratory. The most useful cytologic technique is usually direct brushings of a visible lesion.
PRINCIPLE: To collect an ovarian cyst specimen which will allow for cytologic processing and evaluation.
PRINCIPLE: To collect a pericardial specimen which will allow for cytologic processing and evaluation. Effusions may result from a variety of causes: collagen vascular disease, circulatory disorders, neoplasms, and infections.
PRINCIPLE: To collect a peritoneal specimen which will allow for cytologic processing and evaluation. Effusions may result from a variety of causes: collagen vascular disease, circulatory disorders, neoplasms, and infections.
PRINCIPLE: To collect a pleural specimen which will allow for cytologic processing and evaluation. Effusions may result from a variety of causes: collagen vascular disease, circulatory disorders, neoplasms, and infections.
PRINCIPLE: To collect a sputum specimen which will allow for cytologic preparation and evaluation at the Laboratory. Sputum cytology can be easily obtained and may be useful in the detection of cancer of the respiratory system.
PRINCIPLE: To collect a synovial specimen which will allow for cytologic processing and evaluation.
PRINCIPLE: To collect a thyroid specimen which will allow for cytologic preparation and evaluation at the Laboratory.
PRINCIPLE: In rare instances, a Tzanck smear may be obtained from the skin to determine or aid in the detection of a viral organism that may be present. A properly collected skin scraping or Tzanck smear enables the cytology department to make such a diagnosis.
PRINCIPLE: To collect a urine specimen which will allow for cytologic preparation and evaluation at the Laboratory. Urine cytology is primarily useful in diagnosing diseases that involve the bladder and the collecting tubes that are associated with the bladder.
PRINCIPLE: To ensure proper specimen ordering when collecting a pap smear.
Supplies are available by phone call (x7561) or written request.
1. Each slide must be labeled in pencil with patient name. Name on slide and slip must match in order to prevent rejection of specimen at the Cytology Laboratory. Slide must be labeled with complete first and last name spelling. No abbreviations will be accepted (see diagram 2). Data on the cytology requisition must be answered completely and accurately.

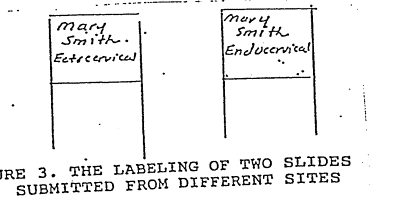
1.In some instances, two slides are submitted on a patient for evaluation. Each slide must be labeled in pencil with patient name and a separate distinction on the slide must be made as to site of each slide. For example, endocervical and ectocervical (see figure 3) write name and site distinction on frosted end of site.
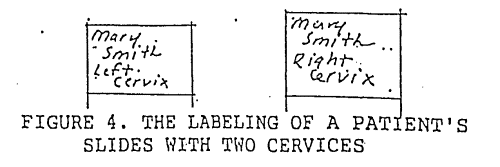
1.In rare instances, a patient will have two cervices. A pap smear should be performed on both cervices. Slides must be labeled in pencil with patient name and a distinction as to left or right must be made (see figure 4). Write name and site distinction on frosted end of slide.
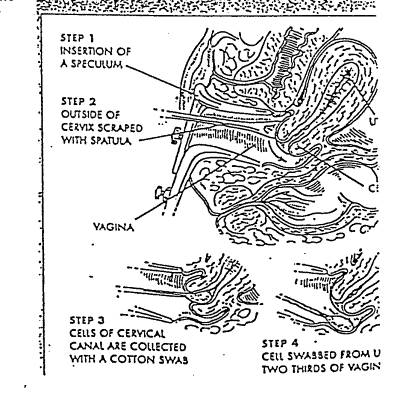
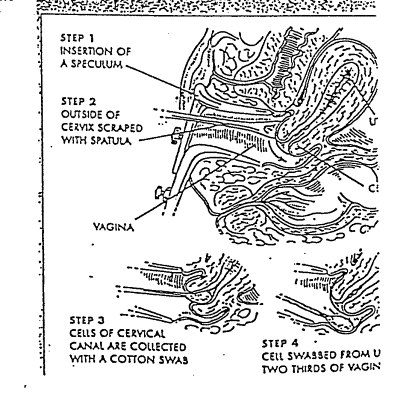
PRINCIPLE: To ensure that a pap smear is properly collected at the doctor’s office.
Method B:
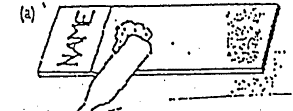
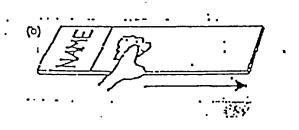
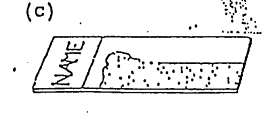
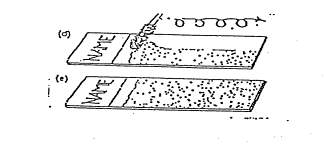
Method C:
Method D (the Maturation Index):
PRINCIPLE: To ensure proper specimen labeling when collecting a pap smear.
PRINCIPLE: To ensure that a ThinPrep pap smear has been collected properly; therefore, the preparation of the specimen will allow for best staining and interpretative results.